Dry Granulation vs. Wet Granulation
Dry Granulation vs. Wet Granulation: A Practical Perspective
Granulation is one of the most critical processes in the development and manufacture of solid dosage forms, particularly tablets. Each method has its own advantages, limitations, and ideal use cases.
What is Granulation?
Granulation is the process of particle size enlargement, where fine powders are agglomerated into larger, multi-particle entities called granules. This improves flowability, compressibility, and reduces dust generation—essential for robust and consistent tablet manufacturing.
DRY GRANULATION: No Liquids Involved Definition
Dry granulation involves the compaction of powders into larger agglomerates without the use of a liquid binder. The compacts (slugs or ribbons) are then milled to obtain granules.
Common Methods
- Slugging – Powders are compressed into large tablets (slugs) using a tablet press.
- Roller Compaction – Powders are compacted between two counter-rotating rollers to form a ribbon, then milled.
When to Use Dry Granulation
- When the API or excipients are moisture-sensitive or heat-sensitive.
- When solvent usage must be avoided due to safety, environmental, or regulatory concerns.
- For high-dose formulations where flow needs improvement but no binder is required.
Advantages
- No drying step – saves energy and time.
- Suitable for thermolabile and hygroscopic materials.
- Simpler validation for moisture-sensitive drugs.
Limitations
- Less densification than wet granulation.
- Not ideal for formulations requiring strong binding.
- Can cause generation of fines during milling, leading to content uniformity issues.
WET GRANULATION: Binding with Liquid
Definition
Wet granulation involves mixing powders with a granulating liquid (usually water or an organic solvent) and a binder to form a wet mass, which is then dried and milled into granules.
Typical Steps
- Mixing of powders (API + excipients)
- Addition of binder solution (e.g., PVP, HPMC)
- Wet massing
- Wet screening
- Drying (typically via fluid bed dryer or tray dryer)
- Milling and sizing
When to Use Wet Granulation
- When the powder blend lacks flowability and compressibility.
- When a stronger granule matrix is needed for uniformity and robustness.
- For low-dose drugs where content uniformity is critical.
Advantages
- Produces dense, uniform granules.
- Excellent for ensuring dose uniformity, especially in low-dose formulations.
- Reduces dust and segregation of components.
Limitations
- Not suitable for moisture- or heat-sensitive drugs.
- Requires more processing steps – time, labor, and energy intensive.
- Needs careful drying to prevent degradation or residual solvent issues.
Dry Granulation vs Wet Granulation: A Practical Comparison
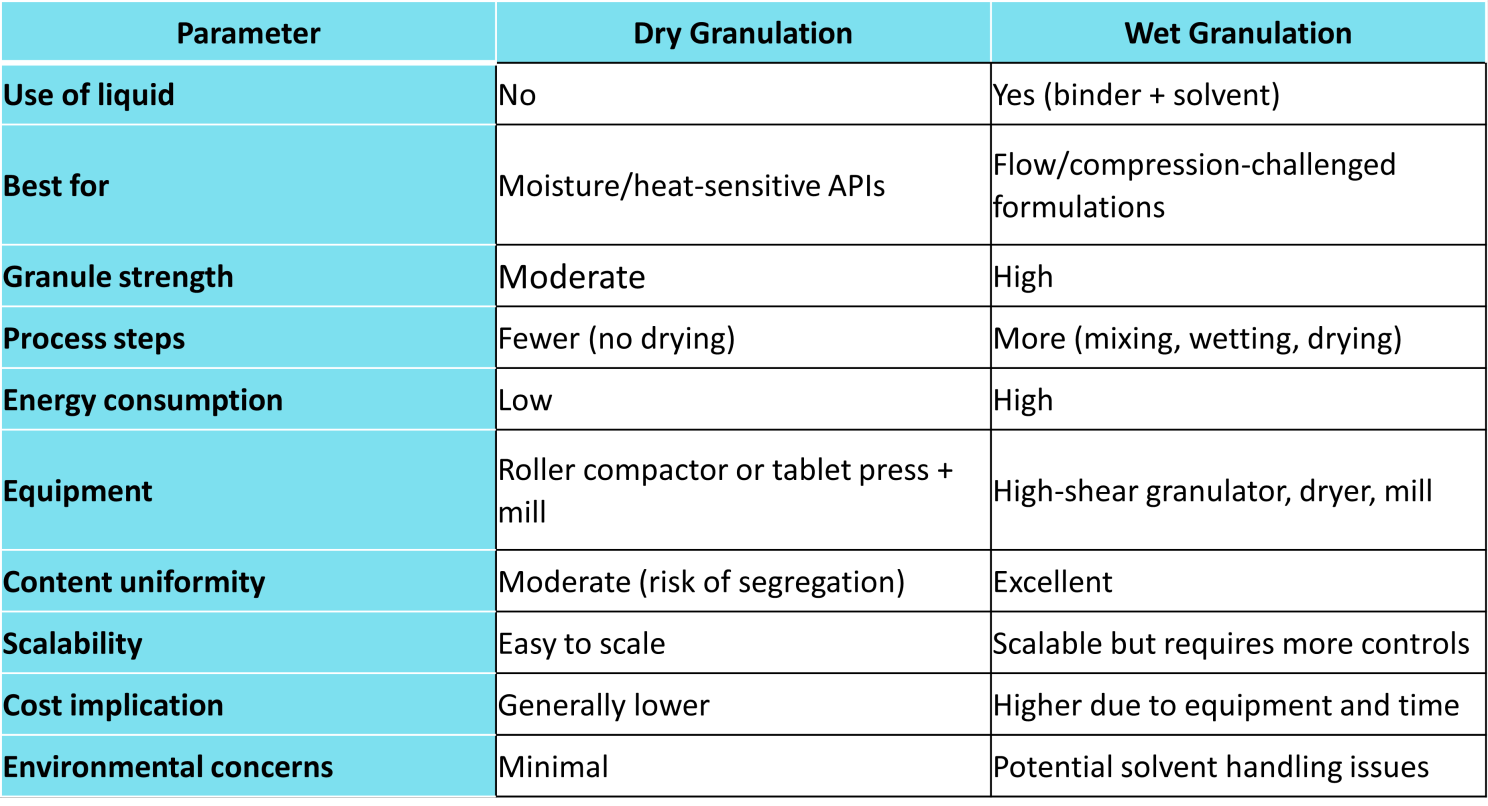
Conclusion
Both dry and wet granulation are essential tools in the pharmaceutical manufacturing toolkit. There is no one-size-fits-all answer—selection should be based on:
- API and excipient properties,
- Target product profile (TPP),
- Process economics, and
- Equipment availability.
A successful granulation process isn’t just about technique—it’s about understanding the material behavior, machine limitations, and the end goal of delivering safe, effective medicines to patients


